Urease Test
The urease test is used to determine the ability of an organism to split urea, through the production of the enzyme urease.
The urease test is used to determine the ability of an organism to split urea, through the production of the enzyme urease.

Principle

Urea is the product of decarboxylation
of amino acids. Hydrolysis of urea produces ammonia and CO2. The formation
of ammonia alkalinizes the medium, and the pH
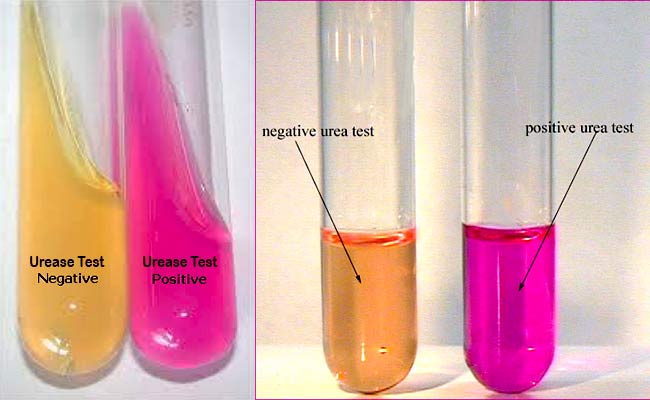
shift is detected by the color change of phenol red from light orange at pH 6.8 to magenta (pink) at pH 8.1. Rapid urease-positive
organisms turn the entire medium pink within 24
hours.
Weakly positive organisms may
take several days, and negative organisms produce no color change or yellow as
a result of acid production.
Uses
1. This test is used to differentiate
organisms based on their ability to hydrolyze urea with the
enzyme urease.
2. This test can be used as part of the
identification of several genera and species of Enterobacteriaceae,
including Proteus, Klebsiella, and
some Yersinia and Citrobacter species,
as well as some Corynebacterium species.
3. It is also useful to identify Cryptococcus spp., Brucella, Helicobacter pylori, and many other bacteria that
produce the urease enzyme.
4. Directly, this test is performed on gastric
biopsy samples to detect the presence of H. pylori.
Media
Composition
Urea
20.0 gm
Sodium Chloride 5.0 gm
Monopotassium Phosphate 2.0 gm
Peptone 1.0 gm
Dextrose
1.0 gm
Phenol Red 0.012 gm
Agar
15.0 gm
Final pH 6.7 +/- 0.2
at 25 degrees C.
Preparation
1. Dissolve the ingredients in 100 ml of
distilled water and filter sterilize (0.45-mm pore size).
2. Suspend the agar in 900 ml of distilled water,
boil to dissolve completely.
3. Autoclave at 121 degree C and 15 psi for 15
minutes.
4. Cool the agar to 50 to 55 degree C.
5. Aseptically add 100 ml of filter-sterilized
urea base to the cooled agar solution and mix thoroughly.
6. Distribute 4 to 5 ml per sterile tube (13 x
100 mm) and slant the tubes during cooling until solidified.
Procedure
1. Streak the surface of a urea agar
slant with a portion of a well-isolated colony or inoculate slant with 1
to 2 drops from an overnight brain-heart infusion broth culture.
2. Leave the cap on loosely and incubate the tube
at 35°-37°C in ambient air for 48 hours to 7 days.
3. Examine for the development of a pink color
for as long as 7 days.
Result

Positive Reaction: Development of an intense magenta to
bright pink color in 15 min to 24 h.
Examples: Proteus spp, Cryptococcus spp, Corynebacterium spp, Helicobacter pylori, Yersinia spp, Brucella spp, etc.
Examples: Proteus spp, Cryptococcus spp, Corynebacterium spp, Helicobacter pylori, Yersinia spp, Brucella spp, etc.
Negative Reaction: No color change.
Examples: Escherichia, Shigella, Salmonella, etc.
Examples: Escherichia, Shigella, Salmonella, etc.
Quality Control
Positive: Proteus vulgaris (ATCC13315)
Weak positive: Klebsiella pneumoniae (ATCC13883)
Negative: Escherichia coli (ATCC25922)
Weak positive: Klebsiella pneumoniae (ATCC13883)
Negative: Escherichia coli (ATCC25922)
References
www.time2026end.com
Comments