Simmons Citrate Agar is an agar medium used for the
differentiation of Enterobacteriaceae based on the utilization of citrate as
the sole source of carbon.
Ingredients per liter of
deionized water:
Principle of Simmons Citrate Agar

Uses of Simmons Citrate Agar
1. It is used for the differentiation of
Gram-negative bacteria on the basis of citrate utilization.
2. Simmons Citrate Agar may be used to
differentiate citrate-positive Salmonella enteritidis and
members of Salmonella subgenus II, III
and IV from the citrate-negative Salmonella typhi, Salmonella
paratyphi A, Salmonella pullorum and Salmonella gallinarum.
3. Simmons Citrate Agar is primarily used to
aid in the identification of Enterobacteriaceae. Uses
include:
include:
- Escherichia coli (usually -) and Shigella spp.
(-) from other commonly encountered
Enterobacteriaceae (variable +). - Edwardsiella spp. (-) from Salmonella spp. (usually
+)
- Serratia proteamaculans (+) from Yersinia pseudotuberculosis (-)
and Yersinia enterocolitica
(usually -) - Klebsiella-Enterobacter groups (usually +) from E. coli (usually -)
- Proteus rettgeri (+) from Morganella morganii biogroups
1 and 2 (-)
- Yokenella regensburgei (+) from Hafnia alvei (-)
- Leminorella grimontii (+) from L. richardii (-)
- Acidovorax delafieldii (+) from A. facilis (-)
and A. temperans (-)
Preparation
1. Dissolve above salts in deionized water.
2. Adjust pH to 6.9.
3. Add agar and Bromothymol blue.
4. Gently heat, with mixing, to boiling until
agar is dissolved.
5. The medium may be used either as slopes in
test tubes or as a plate medium in petri dishes. In both cases the surface of
the medium is lightly inoculated by streaking and, where slopes are used, the
butt of medium is inoculated by stabbing.
6. For tubes, dispense 4.0 to 5.0 ml into 16-mm
tubes.
7. Autoclave at 121 degree C under 15 psi pressure
for 15 minutes.
8. Cool in slanted position (long slant, shallow
butt).
9. Tubes should be stored in a refrigerator to
ensure a shelf life of 6 to 8 weeks.
10. The uninoculated medium will be a deep forest
green due to the pH of the sample and the bromothymol blue.
Culture medium 18-24h

Culture medium 18-24h
subculture on simmons citrate agar tube and streaking an agar slant with the loop.Then take in incubation 37c 18-24h

Result Interpretation
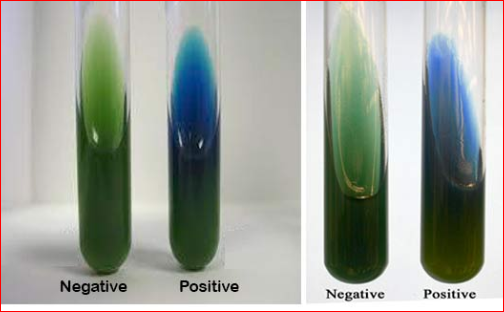
positive growth (i.e. citrate utilisation) produces an alkaline reaction and changes the colour of the medium from green to bright blue.
Negative test (i.e. no citrate utilisation) the colour
of the medium remains unchanged.
Limitations of Simmons Citrate Agar
1. It is important not to carry over any
nutrients into the citrate medium because this will result in false positive
tests.
2. Some organisms are capable of growth on
citrate and do not produce a color change. Growth is considered a positive
citrate utilization test, even in the absence of a color change.
3. Tests with equivocal results should be
repeated.
4. The reactions of this medium alone are not
sufficient for identification to the species level.
5. The inoculum should be from a pure,
overnight culture grown on a solid medium and not from a broth
suspension.
Quality Control on Simmons Citrate Agar
Positive control: Klebsiella pneumoniae ATCC®
13883. Growth; colour change of medium to blue
Negative control: Escherichia coli ATCC® 25922. Inhibited or no growth; no colour change of medium
Negative control: Escherichia coli ATCC® 25922. Inhibited or no growth; no colour change of medium
List of Bacteria which gives positive citrate utilization test
- Klebsiella pneumoniae
- Enterobacter species (minority of strains gives negative result)
- Citrobacter freundii
- Salmonella other than Typhi and Paratyphi A
- Serratia marcescens
- Proteus mirabilis (minority of strains gives negative result)
- Providencia
Citrate Test: variable (different strains give different results)
- Proteus vulgaris
- Vibrio cholerae
- Vibrio parahaemolyticus
Citrate test: negative
- Escherichia coli
- Shigella spp
- Salmonella Typhi
- Salmonella Paratyphi A
- Morganella morganii
- Yersinia enterocolitica
Although uncommon, natural E. coli variants that are citrate positive have been isolated. Citrate-negative strains of E. aerogenes have also been found.
References
www.time2026end.com
www.time2026end.com

Comments