It used to differentiate among the Gram-Negative bacilli in the family
Enterobacteriaceae.
Principle

Citrate agar is used
to test an organism’s ability to utilize citrate as a source of energy. The
medium contains citrate as the sole carbon source and inorganic
ammonium salts (NH4H2PO4) as the sole source of nitrogen.
Bacteria that can grow
on this medium produce an enzyme, citrate-permease,
capable of converting citrate to pyruvate. Pyruvate can
then enter the organism’s metabolic cycle for the production of energy. Growth is indicative of utilization
of citrate, an intermediate metabolite in the Krebs cycle.
When the bacteria
metabolize citrate, the ammonium salts are broken down to ammonia, which increases alkalinity. The shift in pH turns the bromthymol blue indicator in the medium from green to blue above pH 7.6.
Christensen developed an alternative citrate test
medium that does not require the organism to use citrate as a sole carbon source. Christensen’s
medium contains both peptone and cysteine.
Thus citrate-negative bacteria can also grow on this medium. A positive
reaction shows that the organism can use citrate but not necessarily as the
sole carbon source.
Media

Sodium Chloride 5.0 gm
Sodium Citrate (dehydrate)
2.0 gm
Ammonium Dihydrogen Phosphate 1.0 gm
Dipotassium Phosphate 1.0 gm
Magnesium Sulfate (heptahydrate) 0.2 gm
Bromothymol Blue 0.08 gm
Agar 15.0 gm
Deionized water
= 1,000 ml
Final pH 6.9 +/- 0.2 at 25 degrees C.
Preparation
1. Dissolve above salts in deionized water.
2. Adjust pH to 6.9.
3. Add agar and Bromothymol blue.
4. Gently heat, with mixing, to boiling until
agar is dissolved.
5. Dispense 4.0 to 5.0 ml into 16-mm tubes.
6. Autoclave at 121 degree C under 15 psi
pressure for 15 minutes.
7. Cool in slanted position (long slant, shallow
butt).
8. Tubes should be stored in a refrigerator to
ensure a shelf life of 6 to 8 weeks.
9. The uninoculated medium will be a deep forest
green due to the pH of the sample and the bromothymol blue.
Procedure
1. Streak the slant back and forth with a light
inoculum picked from the center of a well-isolated colony.
2. Incubate aerobically at 35 to 37C for up to
4-7 days.
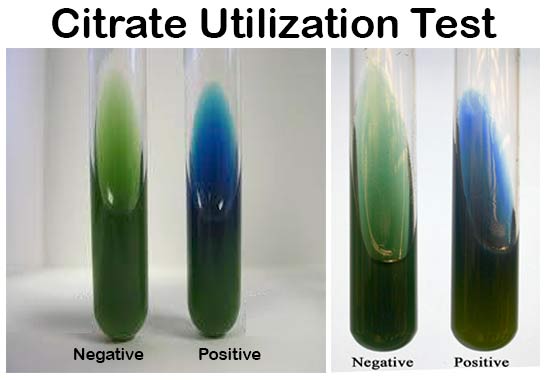
3. Observe a color change from green to blue
along the slant.
Result

Positive Reaction: Growth with color change from green to
intense blue along the slant.
Examples: Salmonella, Edwardsiella, Citrobacter, Klebsiella, Enterobacter, Serratia, Providencia, etc.
Examples: Salmonella, Edwardsiella, Citrobacter, Klebsiella, Enterobacter, Serratia, Providencia, etc.
Negative Reaction: No growth and No color change; Slant
remains green.
Examples: Escherichia, Shigella, Morganella, Yersinia etc.
Examples: Escherichia, Shigella, Morganella, Yersinia etc.
Quality Control
Citrate Positive: Klebsiella pneumoniae ATCC
13883 (growth; blue color)
Citrate Negative: Escherichia coli ATCC 25922 (no growth or trace of growth)
Citrate Negative: Escherichia coli ATCC 25922 (no growth or trace of growth)
References
www.time2026end.com
Comments